Equilibrium Equations- Using Keq To Solve Problems | Part One Chemistry 30 Video Tutorial Solving Equilibrium Expressions: Using Keq To Solve Problems #EquilibriumExpressions #Chemistry #VideoTutorial... | By TutorTag | Facebook

calculating Keq Wkst - Worksheet: Calculating Keq Name______________ CHEMISTRY: A Study of Matter © - Studocu
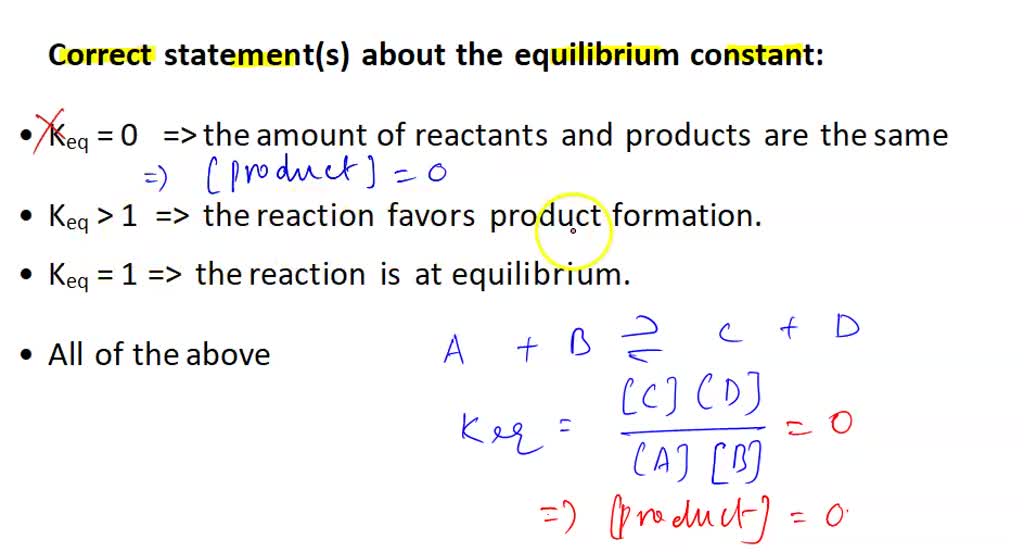
SOLVED: Which of the following statements about the equilibrium constant is correct? Keq = 0 the amount of reactants and products are the same Keq " >1 the reaction favors product formation.